- Published:
- 16 October 2025
- Author:
- Professor Sharon Brookes and Dr Fabian Lean
- Read time:
- 12 Mins
Veterinary pathologist Dr Fabian Lean and virologist Professor Sharon Brookes report on avian influenza viruses, explaining how they spread among birds and transmit to humans and other animals, leading to significant economic and health impacts. They also explain how surveillance can help to control and potentially eliminate bird flu altogether.
Avian influenza is a highly contagious disease caused by avian influenza viruses (AIVs) in birds, both domestic and wild species. The virus is capable of transmission to mammals, including humans, and can cause clinical disease when infection is established.
AIVs are a broad group of segmented RNA viruses characterised by their haemagglutinin (H) and neuraminidase (N) surface antigens, which can be divided into subtypes. There are 16 H-types and 9 N-types that make up both the low- and high-pathogenicity viral pathotypes (LPAIV and HPAIV, respectively).1 The HPAIV bird flu viruses are either H5 or H7 viruses with a range of N-types, such as H5N1, H5N8 and H7N9 lineages.
Wild birds, mostly wild waterfowl, can be the reservoir for LPAI viruses; such infections are not generally associated with disease or mortality in their hosts. However, some of these LPAI viruses can be transmitted into poultry through direct or indirect transmission. If this is followed by genetic mutation, adaptation, transmission and dissemination, some of those viruses can mutate to become HPAI viruses, which results in severe clinical disease and domestic bird losses.
Historically, HPAI viruses have not been transferred back into wild waterfowl; these wild aquatic birds have not had substantial involvement in the spread of HPAI to poultry or other domestic birds. However, in recent years, the epidemiology of HPAI viruses has changed. They have become endemic in domestic birds in several countries, causing major outbreaks but also killing many wild bird species worldwide, some in considerable numbers. As a result, this has facilitated the spread of the virus along established global wild bird migratory routes, resulting in the death of many birds, including conservation species. The number of disease outbreaks continues to act as a source for virus transmission to poultry, wild and domestic mammals, and people.2
The AIVs with zoonotic potential for transmission to humans include both HPAIVs (H5Nx and H7Nx) and LPAIVs (H3N8, H5Nx, H6N1, H7Nx, H9N2, H10Nx and H11 LPAVs).3 The pandemic potential of these viruses requires enhanced vigilance, especially in animal populations where animal-to-animal transmission is known to occur, and close monitoring in animals and humans.
The most recent cases in wild birds, poultry and mammals including humans are due to the influenza A(H5) clade 2.3.4.4b (lineage) of subtype H5N1 virus, most notably seen throughout the USA in birds, cattle, cats and people, plus birds and a sheep in the UK.4,5
‘Bird flu’ refers to the clinical disease caused by AIV infection in birds. A common misconception is that infected birds develop flu-like respiratory symptoms similar to those seen in humans. In reality, the clinical disease presentation varies by virus strain. LPAI virus infection usually causes mild respiratory or reproductive clinical signs in poultry.
In contrast, HPAI virus infection, particularly certain H5 and H7 subtypes, leads to acute systemic disease with high mortality. Affected birds may show signs such as respiratory distress, diarrhoea, cyanosis, neurological signs and sudden death.6
Since 2021, HPAI has evolved from a regional concern affecting poultry to a global threat to avian, mammalian and human health. Since 2005, the Goose/Guangdong HPAIV lineage has driven multiple intercontinental epizootics. Migratory wild birds play a key role in long-distance virus dissemination, enabling seasonal virus incursions across Europe, Asia, Africa and the Americas. The most recent bird flu cases in wild birds, poultry, and mammals including humans are due to the aforementioned influenza A(H5) clade 2.3.4.4b (lineage) of subtype H5N1 virus.4–7
The March–April 2025 global situation reports4,8,9 indicated that, since October 2024, HPAI disease had been reported as 142 outbreaks in poultry and 214 in non-poultry birds and in mammals in the Americas, Asia and Europe. The 6 months from October 2024–March 2025 saw an increase (to 1,332 outbreaks) above that for the previous wave (1,062 outbreaks). In the UK, HPAI has led to repeated outbreaks in poultry and wild birds, particularly during the winter months, but there have been exceptions in the recent years. In the summer of 2021, there were die-offs in seabird colonies (gannets, skuas, seagulls) that marked a concerning change in epidemiology.10
Wild birds
Wild aquatic birds or anseriformes (ducks, geese, swans) are the conventional reservoir hosts of AIVs, and infection of these birds typically results in subclinical disease (LPAI). However, recent HPAIV strains have caused systemic disease and mortality in these non-domestic species sporadically.
The global distribution of HPAI is heavily influenced by the migratory behaviour of wild birds. As these birds move along established flyways, they facilitate long-distance dissemination of the virus. Where major migratory pathways overlap, as in Northern Europe, East Asia and parts of Africa and the Americas, there is increased risk of viral mixing and cross-infection among wild populations, generating reassortant virus (mixing of the RNA genome segments of 2 or more viruses in 1 cell), and spillover into domestic poultry.
Poultry
In birds, AIVs are shed in the faeces and respiratory secretions. They can all be spread through direct contact with secretions from infected birds, especially through faeces or through contaminated feed and water. Because of the resistant nature of AIVs – including their ability to survive for long periods when temperatures are low – they can also be carried on farm equipment and spread easily from farm to farm.2
In poultry settings, HPAI outbreaks are frequently associated with lapses in biosecurity. A recent UK case investigation highlighted how structural deficiencies – such as gaps in housing – enabled wild birds, including free-ranging pheasants and scavenging bridge species like gulls and corvids, to access feed inside poultry sheds (see Figure 1).11 While this example illustrates just one scenario, it underscores how inadequate exclusion measures can compromise flock protection. To reduce the risk of virus introduction during high-risk periods, the UK government may implement housing orders requiring all poultry to be kept indoors when faced with immense infection pressure from the environment.
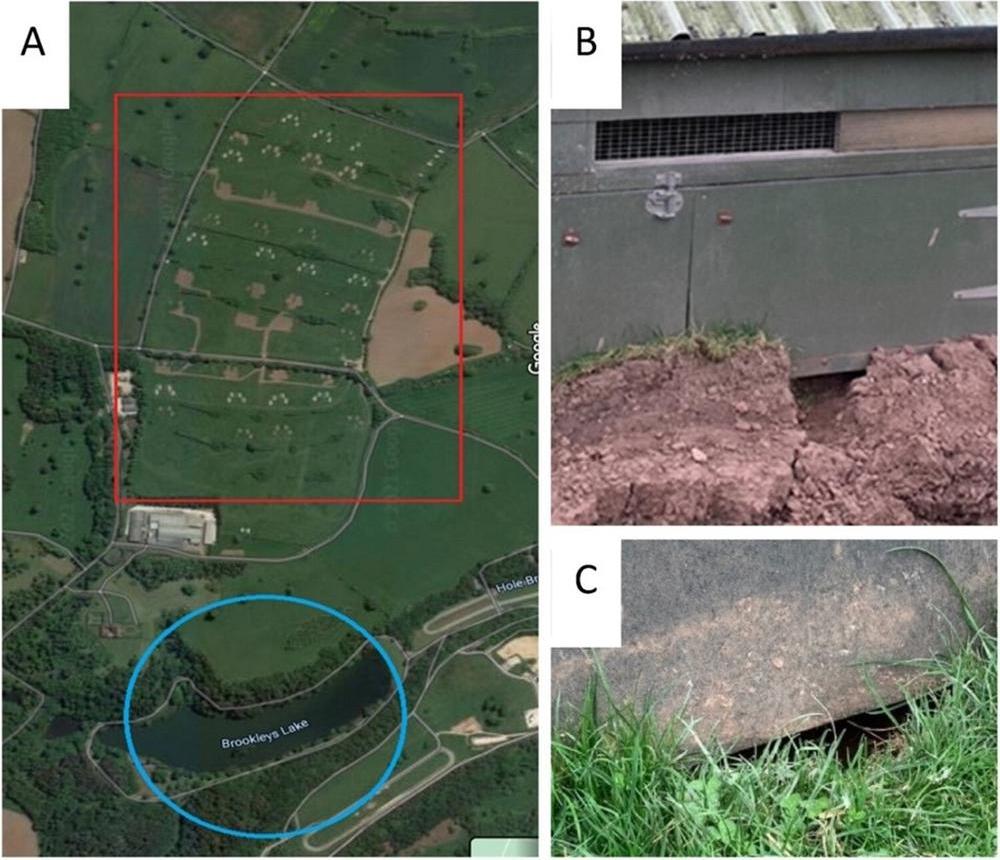
Figure 1. Ecological context of the site (A) (red rectangle denotes pasture, blue circle highlights the small lake) including evidence for wild animal and wild bird activity around poultry houses (B and C).11
© 2024 The author(s). Published by Informa UK Limited, trading as Taylor & Francis Group, on behalf of Shanghai Shangyixun Cultural Communication Co., Ltd. CC by 4.0.
Terrestrial and marine mammals
Avian influenza spillover into mammals is being increasingly documented across the globe. Terrestrial species, including foxes, raccoons and wild and domestic cats – as well as marine mammals, such as seals and sea lions – have been infected, often fatally, after ingesting infected birds or being exposed to contaminated environments. These spillover events are frequently linked to high environmental contamination from wild bird die-offs caused by HPAIV. While carcass collection can help mitigate this risk, large-scale outbreaks across remote or multiple locations make effective control logistically difficult.12
In 2024, HPAI H5N1 was detected in dairy cattle across several American states, marking a significant host shift. Although the initial source of transmission remains uncertain, sustained cow-to-cow transmission has been identified. Affected cattle showed respiratory clinical signs, reduced milk yield and visibly abnormal milk. While limited human infections have occurred among dairy workers, likely consequent to exposure on farm, the virus currently appears to be largely restricted to bovine hosts.7
Humans and the One Health approach – infection and food sources
The risk of bird flu transmission to human health continues to be low for the general public. The risk of infection for those occupationally exposed is low-to-moderate, sporadically occurring following close and repeated exposure and depending on the mitigation measures in place and the local avian influenza disease context. Although additional human infections associated with exposure to infected animals or contaminated environments are expected to occur, the overall public health impact of such infections at a global level, at the present time, is considered minor.4
Veterinary pathologists are integral to avian influenza surveillance, outbreak response and experimental research. Their work spans the range from gross examination of submitted carcasses to detailed histopathology and immunohistochemistry, correlating findings with molecular data to provide insights into disease mechanisms and transmission dynamics.
Necropsy remains a frontline diagnostic tool in avian influenza surveillance, allowing early detection of characteristic lesions and guiding targeted sampling for laboratory testing (see Figure 2). During the 2020–2022 UK outbreaks,13 our group consistently observed HPAIV-associated lesions, including pancreatic and splenic necrosis and multi-organ haemorrhage. However, lesion recognition can be challenging in atypical or rarely examined avian species.

Figure 2. Necropsy conducted in microbiology cabinet in high biological containment laboratory.
There may also be health and safety risks during necropsy, particularly when handling raptors or other birds with sharp talons or manipulating larger birds in a space-restrictive microbiological cabinet (see Figure 3). Nevertheless, early findings from necropsy play a critical role in shaping subsequent laboratory investigations and informing real-time disease control decisions by the chief veterinary officers.

Figure 3. Chicken feet, extensive ecchymosis.
Pathologists also play a crucial role in developing animal models to study viral pathogenesis. For instance, in a comparative study of H5N1-2020 and H5N8-2020, the latter caused faster systemic spread and more severe lesions in chickens; these findings were consistent with field necropsies and were valuable for outbreak risk assessment (see Figure 4).14 The specialist training also enables them to distinguish induced lesions from background pathology, especially in non-specific pathogen-free or outbred species like gamebirds. In addition, pathologists contribute to zoonotic risk assessment. In a recent study,15 a reassortant H9N9 virus (derived from H7N9 and H9N2) was evaluated in ferrets. Pulmonary pathology and viral distribution confirmed the virus’s potential to cause respiratory disease in mammals.
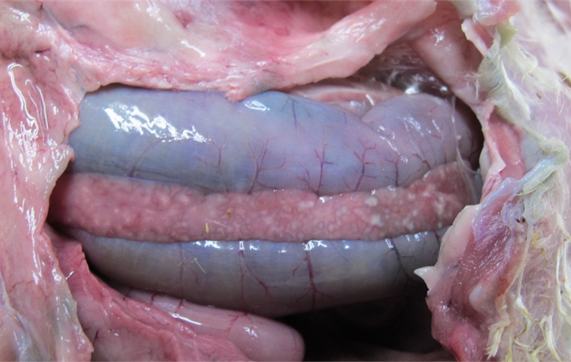
Figure 4. Turkey, pancreatic necrosis.
© 2021 Crown copyright. Veterinary Record published by John Wiley & Sons Ltd on behalf of British Veterinary Association. CC by 4.0.14
The first line of defence against avian influenza is early detection through surveillance and reporting.16 Putting in place accurate warning systems is thus essential to efficiently prevent and control the disease. Because of its capacity to rapidly spread across regions, early detection and timely reporting of cases are key to enable countries to anticipate and get prepared for potential new outbreaks of avian influenza. Avian influenza is a World Organisation for Animal Health (WOAH)-listed disease. For this reason, national veterinary authorities must report:
- infection with HPAIVs, irrespective of their subtypes, detected in birds (domestic and wild)
- infection of birds other than poultry, including wild birds, with influenza A viruses of high pathogenicity
- infection of domestic and captive wild birds with LPAIVs that have proven natural transmission to humans associated with severe consequences.
When LPAI viruses are detected in wild birds, countries can voluntarily report them through the voluntary report on non-WOAH-listed diseases in wildlife. In addition, countries may self-declare the absence of HPAI from their territory on a voluntary basis.
Biosecurity measures are crucial to protect flocks from infection, and to mitigate transmission to other species and the economic and health impacts of outbreaks.17 These mitigations include the following:
- restrict access to poultry premises: this includes limiting visitors, prohibiting access of vehicles to poultry sheds and not sharing farm workers across farms
- improve hygiene: handwashing after contact with birds, disinfecting footwear dips, and using appropriate personal protective equipment (PPE) are essential
- isolate and treat sick birds: early detection and treatment of sick birds can help prevent the spread of infection
- limit wild bird access: preventing wild birds from entering poultry sheds reduces the risk of introducing the virus
- clean and disinfect: regularly cleaning and disinfecting premises, equipment, and vehicles helps to eliminate the virus and prevent its spread
- surveillance: monitoring for dead wild birds and wild mammals helps to detect and track the spread of avian influenza
- compliance with regulations: following government regulations and requirements regarding biosecurity, such as those in the avian influenza prevention zone, is crucial.
Control strategies for HPAIV bird flu include elimination of infected birds or, in some limited circumstances, vaccination.18
Elimination
When an infection is detected in poultry, a policy of culling infected animals and the ones in close contact is normally used in an effort to rapidly contain, control and eradicate the disease. Selective elimination of infected poultry, movement restrictions, improved hygiene and biosecurity, and appropriate surveillance should result in a significant decrease of viral contamination of the environment. These measures should be taken whether or not vaccination is part of the overall strategy.
Systems of financial compensation for farmers and producers who have lost their animals as a result of mandatory culling ordered by national veterinary authorities vary around the world; unfortunately, they may not exist at all in some countries. The WOAH encourages its members to develop and propose compensation schemes because they are a key incentive to support early detection and transparent reporting of animal disease occurrences, including avian influenza.
Vaccination
Under certain specific conditions, vaccination of poultry may be recommended.19 However, this measure alone should not be considered a sustainable solution to control avian influenza. It must be used as part of a comprehensive disease control strategy, in addition to other health measures. The vaccines used should comply with the standards described in the WOAH Terrestrial Manual. Vaccination will not affect the HPAI status of a free country or zone if surveillance supports the absence of infection.
The decision to set up vaccination plans rests with the veterinary authority of each country. It must be based on a risk analysis at regional and national level and in consideration of the international context, potential economic consequences of current outbreaks, and the capacity of the veterinary services to conduct an effective vaccination campaign.
Avian influenza outbreaks can have heavy consequences for the poultry industry, the health of wild birds and farmers' livelihoods, as well as international trade. Farmers might experience a high level of mortality in their flocks, with rates often around 50%, or 100% where elimination is imposed by authorities. Job losses in developing countries can be significant due to the labour-intensive nature of the poultry industry. The presence of HPAI also restricts international trade in live birds and poultry meat, which can heavily impact national economies.20
Avian influenza poses little risk through effective food safety practices (eggs, poultry products, dairy cattle products) and agricultural policies, though large-scale outbreaks can significantly impact consumer confidence. The elimination of infected and at-risk poultry flocks, plus disrupted supply chains, leads to reduced availability of poultry products and occasional shortages in supermarkets. Carcasses and products from bird flu outbreaks must be destroyed and not be fed or allowed to be consumed by other animals to limit cross-species infections.
Global bird flu outbreaks by AIVs continue to be a significant disease burden for animals and pose a zoonotic threat to humans and, as such, are placed high on any country's disease risk register and will continue to be for the foreseeable future.
References available on our website.