- Published:
- 16 October 2025
- Author:
- Dr Duaa Saeed-Chesterman
- Read time:
- 9 Mins
In this article, Dr Duaa Saeed-Chesterman explains the development of the NHS Cervical Screening Programme, highlighting its success in reducing cervical cancer rates through human papillomavirus (HPV) testing and vaccination, and outlines plans to harness emerging technologies like molecular triage and digital pathology to work towards NHS England’s target of eradicating cervical cancer by 2040.
Cervical cancer cases in the UK are decreasing but the disease continues to be a major public health issue. Cervical cancer is the 2nd most common cancer among women and people with a cervix under 35 years of age1 and is the 14th most common cancer in females in the UK.2
The advent of the NHS Cervical Screening Programme (NHSCSP) in 1988 introduced a call–recall system inviting women for a cervical smear test, allowing for early detection, monitoring and treatment of cervical dysplasia and cancers. The NHSCSP has resulted in a significant reduction in the number of cervical cancer cases recorded3 and is estimated to save 4,500 lives in the UK each year.4 Recent changes to the screening programme have altered the roles of cytopathology and histopathology in the diagnostic and screening pathways. Further changes reflective of continued research findings in the cervical cancer screening field are also likely to be introduced, with the aim of improving effectiveness and diagnostic accuracy, and reducing the incidence of cervical cancers.
The NHSCSP targets a population of women aged from 25 to 64 for screening. Since June 2025, the screening interval for all women of screening age in England is 5 years, replacing the previous interval of 3 years for those aged 25–49 years. The 5-year interval is dependent on a normal screening result (Figure 1). If the screening test is abnormal, the specimen is assessed by cytology and followed up on separate pathways (see Figures 2 and 3). Patients are returned to routine screening following successful treatment and/or a return to a normal result.
Figure 1
Figure 1: Sample-taking and HPV testing and cytology triage. Contains public sector information licensed under the Open Government Licence v3.0.5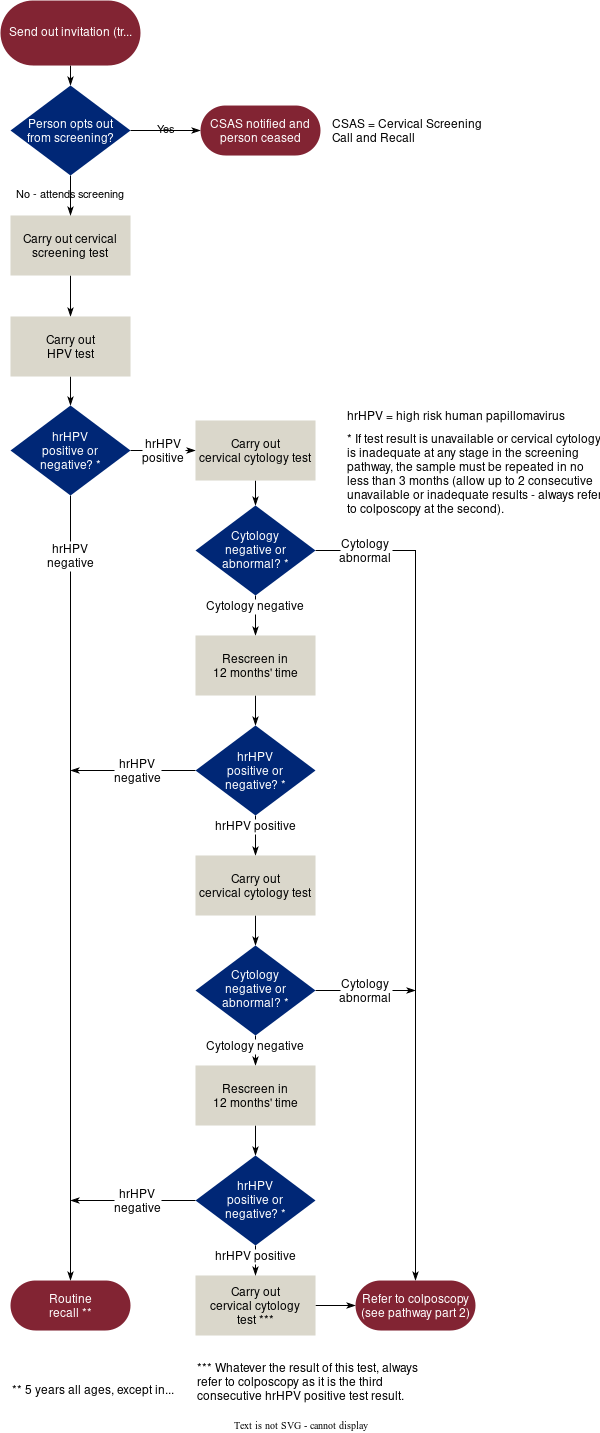
Figure 2
Figure 2: Sample-taking and HPV testing and cytology triage. Contains public sector information licensed under the Open Government Licence v3.0.5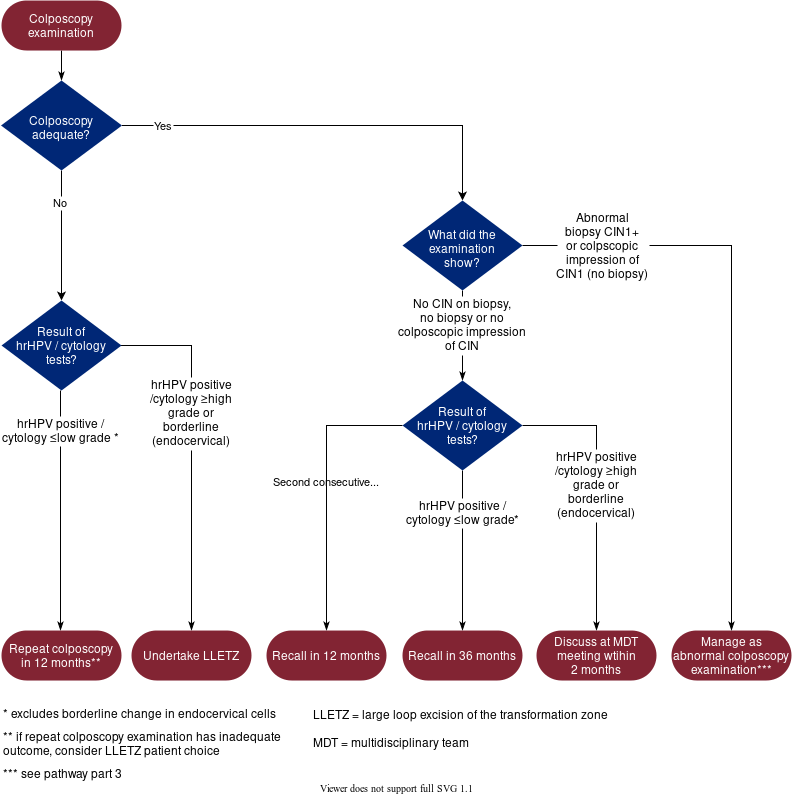
Figure 3
Figure 3: abnormal colposcopy result management (including sample taking, colposcopy and histopathology). Contains public sector information licensed under the Open Government Licence v3.0.5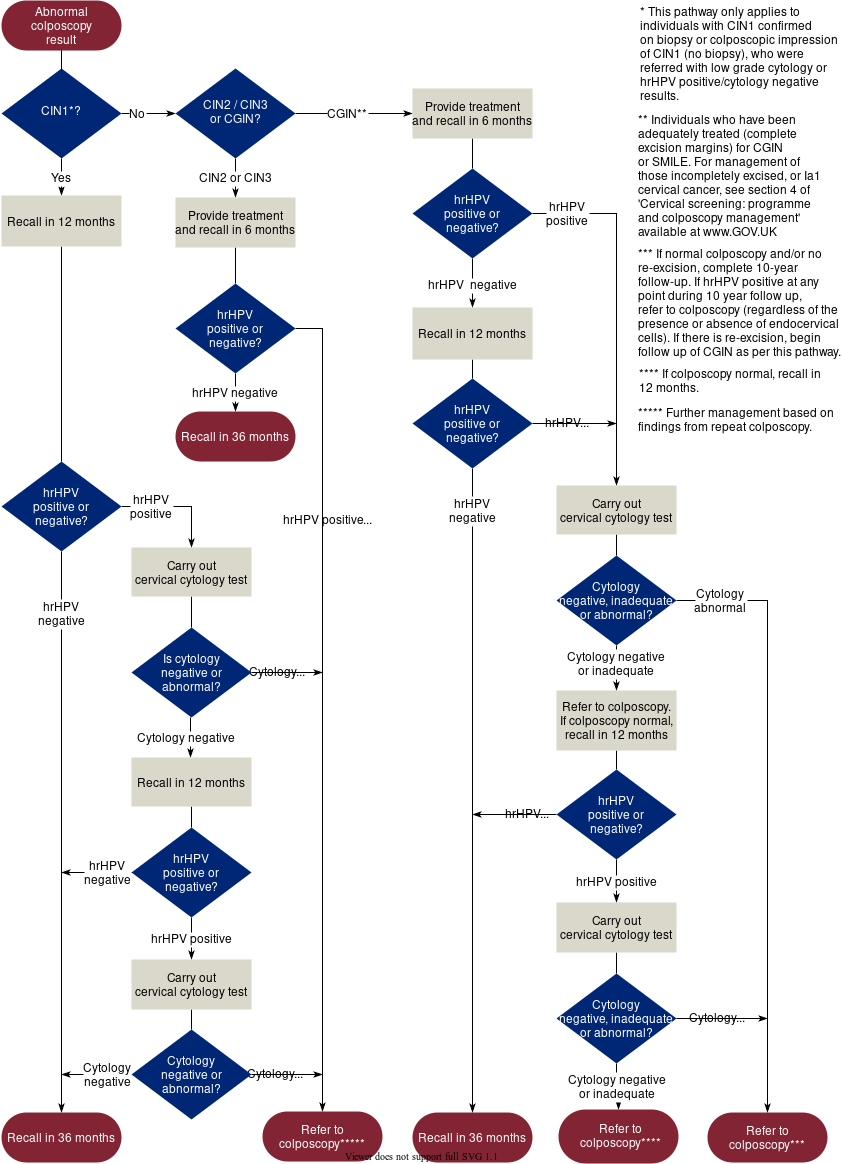
HPV
The vast majority of cervical cancers – approximately 99.7% – are known to be caused by persistent HPV.6 More than 200 HPV subtypes have been identified and are classed as either low- or high-risk based on their oncogenic potential.7 Of the high-risk HPVs (hrHPV), HPV 16 and 18 are considered to be the most important as they are responsible for almost half of high-grade cervical pre-malignancies.7
In the current NHSCSP, genotyping for HPV subtypes other than HPV 16 and 18 is not performed. Evidence of the long-term risk of persistent non-16/18 hrHPV types is lacking, marking an area for potential further research and a scope for the future development of risk stratification based on HPV genotyping.
The methods in which smear tests are assessed within the NHSCSP have evolved over the years, with the conventional Papanicolaou direct smear (more commonly known as the Pap smear) being replaced by the liquid-based cytology (LBC) technique in 2005. The LBC method produces slides with an improved quality of cells, thus improving the accuracy of reporting, in addition to providing the ability to perform reflex HPV testing on the cytologically abnormal smear samples.
Research then emerged showing that hrHPV testing has higher sensitivity and a lower false negative rate than primary cytology testing, allowing for the identification of more patients at risk of developing cervical cancer, at an earlier stage and potentially saving more lives.8 This led to hrHPV becoming the primary screening method from 2019, with reflex cytology being performed on hrHPV-positive patients.
Vaccination
Studies show that the HPV vaccine reduced cervical cancer rates by almost 90% in 20-year-olds who were offered the vaccine at 12–13-years-old.9 The HPV vaccine was rolled out in 2008 in England, to girls aged 12–13, in school year 8. Despite the already significant drop in the incidence of cervical cancers achieved, there is an argument that through the increased uptake of the HPV vaccine and continued cervical screening, the disease could be effectively eradicated. This was echoed in NHS England’s 2023 announcement of a target to eliminate cervical cancer by 2040.10
The major shift into primary HPV testing has resulted in a massive reduction in cytology workload, estimated to be approximately 70%, according to Public Health England. Fewer cytology cases requiring assessment have led to erosion of the cytology screening workforce and a change of role from screening to targeted interpretation. The effect on the workforce was further exacerbated by the centralisation and consolidation of NHSCSP labs, reducing 48 accredited labs to 8 centralised labs as of 2019. Histopathology remains crucial in the diagnosis and management of high-grade lesions and cancer. Cytologists and histopathologists are an integral part of multi-disciplinary team meetings to discuss complex cases requiring a consensus management plan, or to discuss discrepancies between cytology, histology and colposcopy findings with subsequent management formulation.
As a result of the HPV vaccination programme, there has been an expected reduction in the numbers of hrHPV, high-grade cervical intraepithelial neoplasia (CIN) and invasive carcinoma detected. The lower disease prevalence alters the diagnostic workload in cytology and histopathology, resulting in less exposure to abnormal cases and a shift towards lower-grade lesions, potentially reducing diagnostic expertise in the diagnosis of higher-grade lesions. The retention of skills, continued training and maintenance of diagnostic accuracy in the face of decreasing disease prevalence remains a challenge in both cytology and histopathology. Quality assurance and laboratory standards are upheld through participation in cytology and histopathology external quality assessment (EQA) schemes, regular clinical audits and adherence to RCPath Guidelines for the reporting of cervical pathology. This includes a compulsory annual minimum of assessment and reporting of 150 cases per pathologist.
Digital pathology
Digital pathology is being integrated in many cellular pathology departments in the UK and training is provided to staff to enable them to use the software and achieve accreditation in digital reporting. Although the current technology available is not yet suitable for the assessment of cytology specimens, UK Government has now approved the use of digital pathology in histological cancer screening samples. This was a vital step in ensuring that skilled staff who switch to predominantly digital pathology reporting continue to report NHSCSP specimens. This move also ensures that the screening programmes continue to develop in line with emerging technologies. Pilots researching the role of AI-assisted cytology are in progress, with the aim of improving consistency, reducing human error and maintaining performance despite reduced case volumes.
Molecular triage
Developing research has proposed DNA methylation as a possible future molecular triage test that could play a role in cervical cancer screening. By monitoring molecular events in the pathophysiology of HPV infection, it is possible to use DNA methylation to further stratify those with hrHPV into those with disease that has potential to regress and those with disease that is likely to progress to high-grade CIN and cancer.11 The research is still in early stages; however, if this molecular triage is adopted, it has the potential to limit intervention to a particular high-risk group within the group of identified hrHPV-positive patients, providing several clinical benefits. These include a reduction in examinations and invasive procedures for patients who are identified as having hrHPV with regressive behaviour, therefore reducing the number of colposcopy referrals and biopsies and excision specimens submitted to histopathology.
Self-sampling
Efforts to increase uptake of screening have led to an initiative offering self-sampling to women who do not attend their smear test appointment after 6 months from initial invitation. These samples will be tested for hrHPV and, if a positive result is obtained, the patient will be followed up. Although these samples are not suitable for cytological assessments, labs must be prepared to receive and process these specimens when the initiative is rolled out in 2026.
The success of the cervical cancer screening programme is reflected in the continuing decline of the disease burden and was further bolstered by the introduction of the HPV vaccine. The implementation of primary HPV testing improved efficiency but resulted in major changes to the workforce infrastructure, in particular within the fields of cytopathology and histopathology.
To ensure continued effectiveness of the programme and sustainability to achieve its targets, including the NHS England target of eradication of cervical cancer by 2040, there must be constant evolution and innovation applied to the operation and delivery systems. This pertains to several areas, starting from using social media to educate about the importance of cervical screening, increasing uptake of screening and encompassing logistics to ensure retention of skills and integration of emerging technologies.
Adaptation of labs and cellular pathology departments is necessary to accommodate the use of new technologies and molecular tools, and the integration of self-sampling specimens into routine work, and to provide infrastructure support for the evolving workforce requirements. This is a necessity for quality assurance, diagnostic accuracy and continued provision of a high standard of patient care.
References available on our website.




