- Published:
- 17 July 2025
- Author:
- Annette Thomas
- Read time:
- 14 Mins
Point-of-care testing is reaching service users in increasingly diverse settings. In this article, Annette Thomas, Wales National Clinical Lead for POCT, explores how guidance and accreditation can provide quality assurance and safeguard patient safety.
Professional and regulatory guidance on point-of-care testing (POCT)1–4 has provided a welcome and invaluable resource for laboratories developing their POCT services over the last few years. More laboratories are now seeking to gain ISO 15189:20224 accreditation of their POCT services, particularly for a limited scope, such as blood gas and glucose devices in secondary care. However, very few POCT departments are accredited for all equipment in all locations.
Despite professional guidance,5 governance of independently contracted POCT services outside the hospital setting remains challenging.6 Experience in Wales suggests that implementation of quality standards can only be fully achieved through government support and legislation. For example, in Wales, as part of the national POCT strategic plan, the existing national governance policy on POCT7 was recently aligned with the Duty of Quality Statutory Guidance 2023 and Health and Care Quality Standards 2023,8 part of the Health and Social Care (Quality and Engagement) (Wales) Act 2020.9 As a result, and to deliver best practice, POCT users, irrespective of testing location, are required to comply with governance, training, risk management and quality assurance procedures.
POCT departments vary greatly in size across the NHS, from singularly resourced, part-time support within a pathology discipline, to standalone, broadly resourced departments with a POCT manager, co-ordinators, trainers, support staff and a quality manager. Nonetheless, the core role essentially remains the same: to provide audited assurance that the right test and quality are being used for the appropriate clinical purpose, and to ensure that users are trained and competent to safely undertake the test, that limitations are understood and that there is access to personnel that can interpret the test correctly.
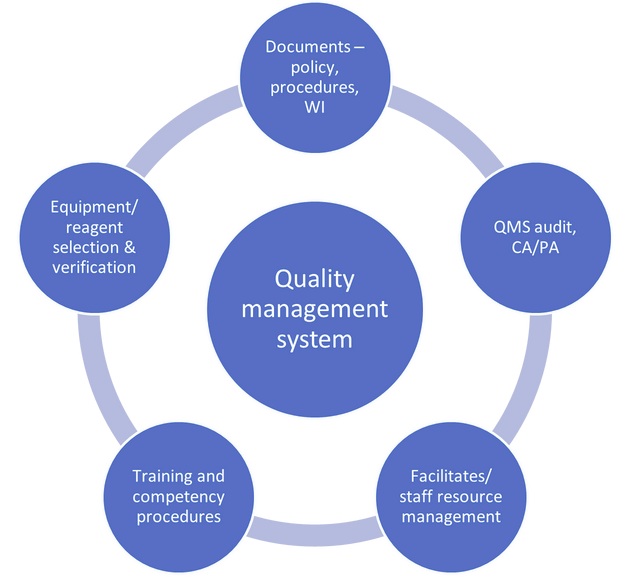
The quality management system (QMS) and quality assurance (QA) tools used within POCT departments are similar to those of accredited pathology laboratories with regard to documents, procedures, equipment selection and verification, QMS audit and QA monitoring tools10 (Figures 1a and b). However, the greatest challenges relate to the lack of direct oversight of the facilities and testing process and the sheer number of different devices and operators undertaking POCT. The very nature of the activity requires that the testing be undertaken outside the laboratory-controlled environment by staff whose primary function is not diagnostic testing. This article attempts to identify these challenges rather than provide a full overview of QA in POCT.
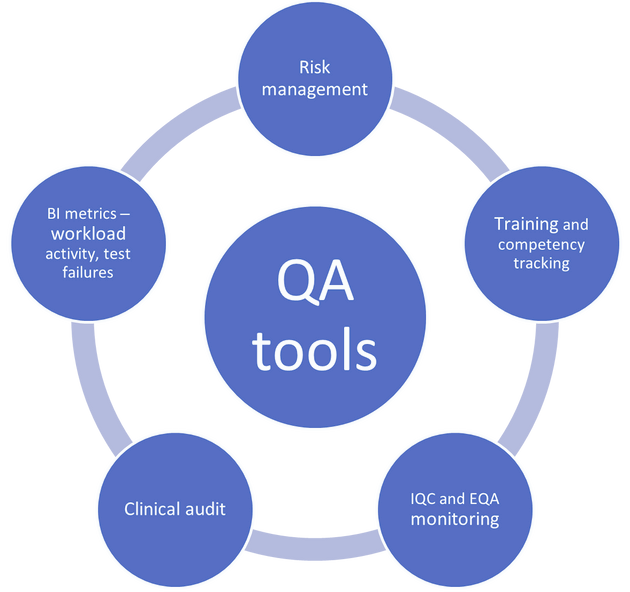
Users should ensure that the implementation of a POCT service or test is safe, timely, effective, efficient, equitable and person-centred. Users should have a sound understanding of the principles of quality assurance (Figure 2).
Figure 2. POCT user QA tools.
Training and competency assessment consideration
To assure quality and safety, only staff that have been adequately trained and assessed as competent should carry out POCT procedures. Realistically, this is not easy, as POCT departments often train and monitor several thousand users of differing grades, from healthcare support workers to consultant clinicians, with varied responsibilities, experience and knowledge across a range of devices.
Using connectivity and POCT data management systems – the ‘middleware’ – is essential to mitigate risk in these high-workload areas. These can be used to manage devices, reagents and operators, including providing dates for training, competency, e-learning and recertification, utilising operator ‘lock-out’ to deliver compliance. Knowledge can be assessed using questionnaires or e-learning, with competencies assessed during probation using observational audits and internal quality control (IQC) monitoring. Large organisations often use cascade trainers and nurse educators to facilitate this. Alternatively, operator competence metrics can be downloaded from the middleware to provide testing patterns, test failures, IQC and external quality assessment (EQA) compliance, and workload data.
As a minimum, training should cover:
- patient preparation
- sample collection and application
- the intended purpose of the device
- the consequences of improper use
- the limitations of use
- reporting and recording of results
- a practical demonstration
- dealing with unexpected results
- maintenance and calibration procedures
- storage of consumables
- IQC and EQA procedures, including frequency and what to do in the event of a failure
- the competence assessment
- the refresher training or e-learning process.
Although the POCT manufacturers/suppliers may also provide training, it is important to ensure that the training covers all of the above and is not solely limited to use of the device.
IQC considerations
When selecting appropriate IQC material for POCT, it is important to consider ease of use, storage conditions and opened vial stability, as well as the concentration range. The choice of a device manufacturer’s supplied, stable liquid artificial matrix, stored at room temperature, may be more appropriate in certain situations than the third-party material preferred by the laboratory. The convenience and greater compliance rate should be balanced against the use of non-commutable material that may miss a potential problem.
There is no universal consensus or guideline on when and how IQC should be analysed; however, a system based on the risk of harm,11 the type and complexity of the device,12 the ease of use and number of patients samples analysed over a specific period offers a sensible solution. The Noklus scoring system proposed for primary care suggests a points-based system assigned for each factor and the total score used to determine the frequency – for example, for high-risk analytes such as HbA1c and complex devices such as blood cell count analysers, a frequency of daily or weekly is recommended. Minimal-risk and simpler devices, such as pregnancy tests, are assigned a monthly frequency. However, the risk of harm of a test depends on the clinical situation and the intervention procedure, as a pregnancy test undertaken before X-ray or a surgical procedure presents a higher risk. Device complexity should not be taken in isolation.
Determining the analytical performance specification
There is no consensus on how the analytical performance specification (APS) should be determined; the belief that the quality of POCT should be the same as that of the laboratory has recently been challenged.13 A less stringent APS is proposed in cases where the requester is familiar with the test’s utility and the clinical situation, or when the test is used for screening or monitoring rather than diagnosis. The APS should be designed to provide the performance that best meets the needs of the service. Care should be taken when using manufacturer-derived IQC limits, as they are often wide and may not fulfil the clinical purpose. Using manufacturer derived limits to set target values and QC lock-out ranges may provide an initial practical solution, especially where large numbers of devices, operators and reagent lot numbers are used in an organisation. These should then be closely monitored using a data management system that allows for further refinement of the limits to reflect clinical need.
External quality assessment
By using dedicated designs, EQA can be used for studying robustness of methods, sensitivity to interferences, linearity, recovery, specificity, and also pre- and post-analytical assessment.14 For the assessment of accuracy, material as close as possible to the patient sample should be used to minimise any matrix effect. This is particularly challenging when the preferred matrix is whole blood, where ease of use and stability may be challenged. Stability can be enhanced through compartmentalisation of the liquid and haemolysate through lyophilisation or addition of stabilisers; however, these present other issues in terms of pre-analytical sample handling and non-commutability. Experience suggests that most users prefer an easy-to-use, stable product.
As with IQC, frequency and the APS should reflect the performance that best meets the needs of the service (clinical relevance), and the clinical risk associated with that investigation.
An essential aspect of EQA for POCT is that reports should be easy to interpret and have sufficient detail to provide the POCT coordinators with information on the performance of their users and devices across the organisation. This information should readily identify poorly performing sites, or sites that have not returned data, and provide statistical tools to help troubleshoot errors and to monitor long-term performance. The POCT users’ reports should be easy to interpret, for example a simple traffic light system with clear actions. Continuous education, training and troubleshooting are pivotal in most well-designed EQA programmes.
Risk management
POCT inherently carries additional risk compared to testing performed within an accredited laboratory, as it is carried out in a busy and distracting environment. Operators have little or no scientific training or experience of QA and no time to reflect before instigating a change in patient management, which combine with the added potential for errors of miscommunication of results.
It is, therefore, important that POCT departments work with POCT users to implement risk management strategies to mitigate potential harm to patients (and healthcare staff) to an acceptable level. These tools typically evaluate the sources of harm (hazards), the potential severity of the harm and the probability of occurring (likelihood), while establishing what control measures are in place to mitigate harm. If the overall risk remains unacceptable, then further options need consideration.7
Table 1 gives an example of using a failure mode effect analysis (FMEA) for a blood glucose meter. The FMEA tool identifies each step in the process and how it could fail. It combines the severity index (SI) and occurrence index (OI) of the failure mode in the process (i.e. the harm) and the ability of any control measures to detect the failure (detection index, DI), generating a risk priority number (RPN).
| Failure mode | Type and potential effect (what could go wrong?) | SI | OI | Control measure (what procedures have I implemented to mitigate risk?) | DI | RPN |
| Identifying the wrong patient | Wrong patient leading to wrong treatment | 5 | 3 |
Electronic positive patient identification on device including name and date of birth prompted by submitted hospital or NHS number Patient identification barcode wrist tag |
1 | 15 |
| Taking an inappropriate sample |
Sample contaminated by:
|
5 | 2 |
Operator understands pre-analytical effects Operator competence regularly assessed |
1 | 10 |
| Incorrect testing procedure |
Incorrect sample volume Incorrect reagents/strips, consumables contaminated or stored at incorrect temperature or humidity Device faulty |
5 | 3 |
Operator trained and assessed as competent Electronic operator lock-out features IQC check of reagent strips and device with automatic IQC lock-out if outside limits. Temperature indicators on reagent boxes Electronic recording of strip information and logging of testing errors |
1 | 15 |
| Incorrect recording of result | Transcription errors due to poor lighting or lack of concentration | 5 | 4 |
Electronic transfer of data to patient medical record Audit trail of date, time and operator associated with test result |
1 | 20 |
| Erroneous results (do not reflect patient's true clinical/biochemical status) |
Analytical interferences:
In-vivo factors:
|
5 | 2 |
Operator trained in limitations of procedure Operator aware of pre-analytical factors on the measurement |
1 | 10 |
| Misinterpretation of the result | Operator misunderstanding the readout of the device | 5 | 2 | Operator trained on device readings and error messages to include units if method is quantitative | 1 | 30 |
| Not acting on the result | Not acting on clinically significant hypo- or hyperglycaemic result | 5 | 4 | Operator trained on critical ranges and when to alert appropriate personnel | 1 | 20 |
Quality assurance for POCT is not an easy task and presents numerous challenges. There is currently no universal consensus on recommendations for the frequency of IQC, frequency of EQA, or recommendations for establishing APS limits. It is hoped that this article will encourage debate in this important area.
References available on our website.




