Rationale for using convalescent plasma
The global pandemic of the new coronavirus SARS-CoV-2 has not just medical, but also historical significance. In many parts of the world, the exponential epidemic curve of cases has overwhelmed hospital services. The primary pulmonary viral infection has required stepped supportive care with oxygen and, if needed, mechanical ventilation.1,2 However, there is currently no specific antiviral therapy that is proven to reduce mortality, although many putative antiviral and anti-inflammatory regimens are being tested in large randomised clinical trials. However, the most promising specific antiviral therapy is also one of the oldest and one where patients who have had COVID-19 can donate plasma that can be used as a therapy for new patients at different stages of the disease.
Convalescent plasma treatment, containing high levels of polyclonal antibody, has been used to treat severe viral pneumonia during previous pandemics. In fact, it has been used intermittently for over a century, including patients treated during the era of the Spanish Influenza pandemic in the early 20th century.3 Although all the studies described at that time had significant methodological flaws and none were randomised, they suggested a reduction in mortality.3
Recently, convalescent plasma has been used to treat H1N1 influenza and, more relevantly, SARS-CoV infections in 2003. In one systematic review there was evidence of some considerable benefit, especially if convalescent plasma was given earlier in the course of the disease (within the first 14 days of symptoms).4
The strategy is to build up a collection of plasma from convalescent donors to provide enough plasma not only for two large-scale randomised controlled trials to assess the efficacy and safety of convalescent plasma, but also to provide enough plasma to treat hospitalised and/or intensive care patients with COVID-19 if the randomised controlled trials do show efficacy.
Convalescent plasma has already been used in observational studies of patients with severe COVID-19. However, a systematic review of the evidence has shown that limited conclusions about the effectiveness and safety of convalescent plasma in people with COVID-19 can be drawn from these studies.5 There have been only eight uncontrolled studies published, with a total of 32 participants. Some of these series are consistent with increased viral clearance and recovery from the disease. A recent report of 5,000 patients treated with convalescent plasma in the USA suggested that convalescent plasma is safe with no obvious cases of antibody-dependent enhancement of disease. However, without data on control patients, there is insufficient evidence to determine whether convalescent plasma is really both safe and effective in the treatment of COVID-19.6
More rigorous assessment of the role of convalescent plasma is underway. At the last count, 22 randomised trials around the world had been registered on trial registries.5 Two living systematic reviews of convalescent plasma will assess the benefits of treating people who have been diagnosed with COVID-19 and the benefits of preventative treatment for people at high risk of getting COVID-19.
Outline of current UK trials
In the UK, NHS Blood and Transplant is leading two large trials of convalescent plasma. This represents a new programme of work funded by the Department for Health and Social Care. The strategy is to build up a collection of plasma from convalescent donors to provide enough plasma not only for two large-scale randomised controlled trials to assess the efficacy and safety of convalescent plasma, but also to provide enough plasma to treat hospitalised and/or intensive care patients with COVID-19 if the randomised controlled trials do show efficacy.
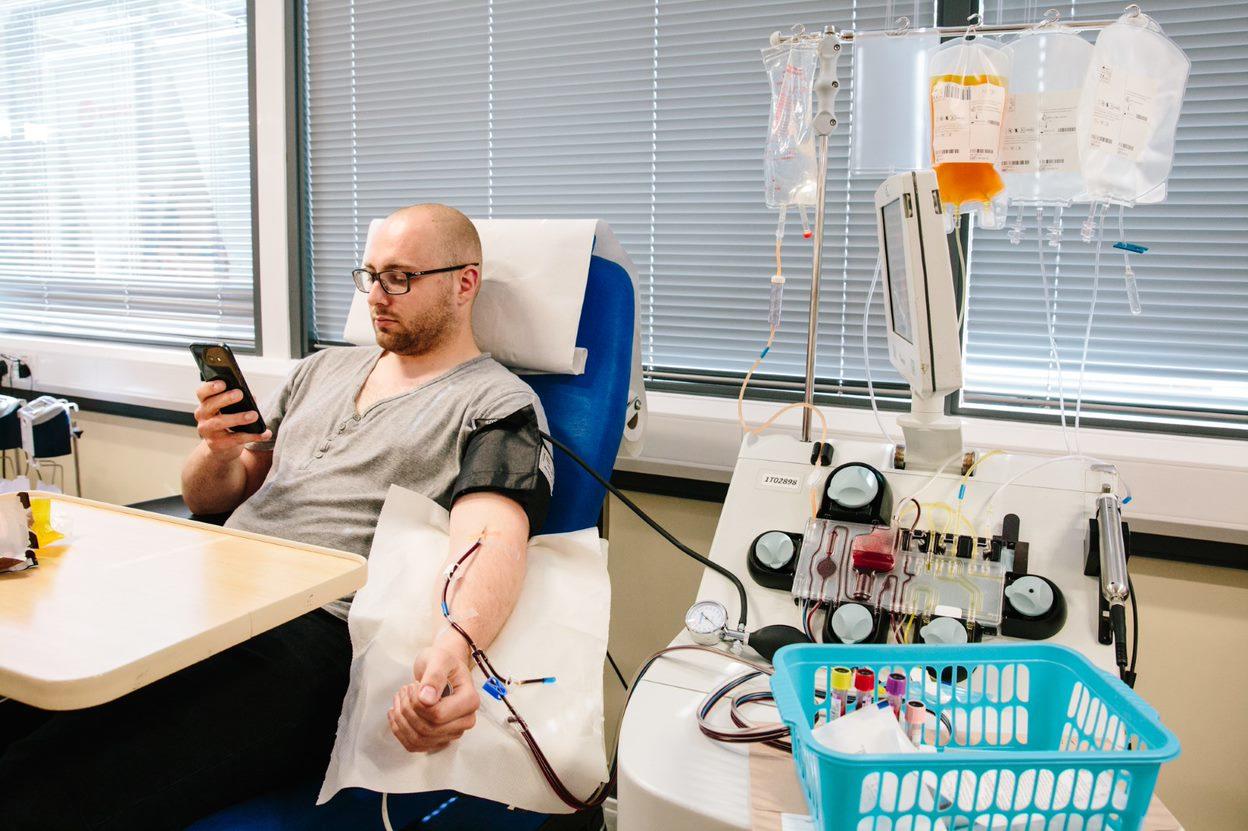
The design of the study has required assessment of the titre of antiviral antibodies using a series of recently developed assays, including neutralising antibody of live SARS-CoV-2 invasion in tissue culture cell, neutralisation of pseudo-typed virus bearing the spike protein from SARS-CoV-2 that contains the ligand for the host ACE2 receptor, and immunological assays of antibodies against different formulations of spike protein.7 Convalescent plasma for clinical trials will only use plasma that has antibodies in the upper third of the range of antiviral antibodies.
REMAP-CAP
The first trial, REMAP-CAP, is for treatment of community-acquired pneumonia in intensive care. This international trial is randomising patients across the UK to several different treatments to assess whether they are beneficial to critically ill adults with COVID-19. One of these randomisations is comparing convalescent plasma with standard care.
It is inevitable that completing any trials of therapy in a pandemic is a race against during the pandemic. These trials have proven no exception.
Patients who have recently been admitted to intensive care are receiving two doses of convalescent plasma to assess whether this decreases the risk of remaining on a ventilator or dying due to COVID-19. Furthermore, any potential harms of this treatment are also being assessed. The plan is to randomise up to 2,000 participants to this trial. This is an adaptive trial and if there is evidence that convalescent plasma improves outcomes for critically ill patients, the trial will be stopped and all patients admitted to intensive care will be given convalescent plasma. The trial is currently open at fifty hospitals around the country and there are plans to open it at more than 100 intensive care units around the country.
RECOVERY
The second trial is a UK-wide trial of convalescent plasma in all hospitalised patients with COVID-19 and this started in May. Patients in the RECOVERY trial receive the same treatment, two doses of convalescent plasma, as in the REMAP-CAP trial. The RECOVERY trial will assess whether convalescent plasma decreases the risk of death or the need for mechanical ventilation for anyone who is hospitalised with COVID-19. It will include people with COVID-19 of any age, including neonates, who are unwell enough to be admitted to hospital. The plan is to randomise up to 5,000 participants to this trial and open the trial at more than 200 hospitals around the UK.
It is inevitable that completing any trials of therapy in a pandemic is a race against during the pandemic. These trials have proven no exception. While the convalescent plasma arms of the REMAP-CAP and RECOVERY trials will be actively recruiting by the end of the May, the number of patients with COVID-19 admitted to hospitals and intensive care units has fallen substantially after several weeks of lockdown. Although many measures are being put in place to control the epidemic, it is almost inevitable that the multiplication of the virus will increase and it seems likely that there will be a long tail of cases and quite probably a resurgence of the infection in the coming months. This will allow completion of the trials and we hope definition of possibly the first, effective therapy for COVID-19.