Genomics in healthcare
Cellular pathology is a rapidly evolving field with a longstanding history of utilising technological advances to improve clinical practices. Genomebased testing now plays an increasingly important role in personalised medicine and healthcare. For safe and effective interpretation of genomic data, integration with traditional cellular pathology is needed. This article briefly outlines the establishment of clinical whole genome testing in NHS England (NHSE) and describes the subsequent curriculum in development to train and upskill the pathology workforce.
The rise of morphomolecular pathology
In a traditional sense, histopathology can be thought of as a morphological specialty, in which pathological entities are identified based on their micro- and macroscopic appearances.1 This has become particularly significant within the field of cancer, in which a combined morphomolecular approach has been shown to enhance diagnostics and result in more personalised treatment.2 For example, genomic profiling in breast cancer has uncovered previously unknown tumour diversity. This has enabled clinically annotated breast cancer tumours to be stratified into novel integrative subgroups that are associated with different prognostic and therapeutic implications.3
Genomic testing in NHSE
Whole genome sequencing (WGS) is now part of the mainstream clinical care offered by NHSE’s new Genomic Medicine Service (GMS). The NHS is the first healthcare system to offer clinical WGS as a routine diagnostic test, and by 2023/2024 the GMS aims to sequence 500,000 whole genomes in genetically mediated diseases, including rare diseases, infectious diseases and cancer.4
The GMS brought to fruition eight years of national infrastructure development to embed genomics as part of routine care in England’s health service.5 Set out by the UK government and building on the 100,000 Genomes Project, the vision for the next ten years is for the UK to remain a global leader in genomic healthcare through collaborations between a range of healthcare experts, government groups, charities, and patient, research and technology communities. This vision will be delivered by focusing on three areas including diagnosis and personalised medicine, prevention and research.4
Interpretation of genomic findings in diagnosis and personalised medicine
An understanding of the genetic determinants in patients with genetically mediated diseases will result in improved diagnoses and personalised treatments. As cancer is primarily an acquired disease of the genome, integrating morphological and genomic data is essential for its diagnosis. Furthermore, the expansion of genomic-enabled personalised medicine and risk-stratified screening will bring great benefit to UK cancer patients. With WGS of cancer samples now possible in clinical practice, the integration of traditional morphology with molecular genomic data is becoming a vital aspect of pathology reporting.
Akin to the well-established model of multidisciplinary meetings, genomic meetings specifically serve to ensure that genomic data, alongside the health data of the patient, have been integrated with specialist knowledge.
The majority of molecular tests requested by doctors are interpreted by a clinical scientist or medically qualified pathologist. It follows that interpretation of genomic data may also require specialist discussion of potential clinically actionable (diagnostic, prognostic or therapeutic) rare variants. As part of the GMS, a model for genomic meetings has been established.6 Akin to the well-established model of multidisciplinary meetings, genomic meetings specifically serve to ensure that genomic data, alongside the health data of the patient, have been integrated with specialist knowledge. Genomic meetings need to quorate with the attendance of molecular pathologists, haematologists or pathologists from relevant specialties to ensure genomic findings are suitably considered and rapidly implemented into routine care.
Curriculum development and proposed models for training pathologists
Key components of molecular diagnostics that need to be encompassed in genomic-focused educational initiatives include knowledge of genomic technologies, data interpretation, an understanding of certain biological pathways and the ability to assess the clinical relevance of test results.3 Changes in the preparation of samples to ensure the validity of genomic analysis will also need to be addressed. Formalin causes severe DNA degradation, while DNA extracted from fresh tumour tissue yields higher quality results. Therefore, future practices are moving towards the collection of fresh tissue as a standard of care in cancer diagnostics, with an aim to produce optimal and consistent genomic results.5 This brings with it several challenges, including the development of new techniques, with pathologists playing a vital role in enabling this transformation.
It became quickly apparent that the curriculum expectations were aspirational and challenging in terms of delivery of training.
The pathology community and the Royal College of Pathologists (RCPath) have anticipated the need for curriculum changes to equip pathologists in the genomic era. The 2010 version of the RCPath histopathology curriculum included an expectation that all histopathology trainees would have an entry-level understanding of the practice of molecular pathology and genomic medicine.7 In addition to the theoretical knowledge, there was a requirement for an understanding of sample preparation and assessment of cellularity.
These guiding principles were further expanded and developed for the current 2015 version of the curriculum.8 Workplace-based assessments have been recommended for inclusion in each stage of FRCPath training, with questions based on molecular pathology and genomics in Part 1 and Part 2 examinations.
It became quickly apparent that the curriculum expectations were aspirational and challenging in terms of delivery of training. Many consultant trainers were appointed before genomic testing became widely available and were reluctant to provide in-house training because of a lack of confidence or knowledge, or both. Furthermore, some testing was undertaken in specialist laboratories that were not easy for trainees to access.
Following the curriculum update in 2015, the RCPath Specialty Training Committee, with support from the National Cancer Research Institute’s Cellular Molecular Pathology workforce and training workstream, produced a paper with a recommendation of a two-week-long placement in a molecular pathology laboratory environment, ideally taken at assessment ST2.9 The paper outlined learning expectations that covered the entry-level curriculum requirements. Vitally, the concept of the laboratory attachment has been accepted across the training programmes in the UK.
There is debate as to how molecular pathology is practised and at what level, with several models proposed for future training programmes.
An alternative academic route is through the offering of university Masters programmes in molecular pathology. However, placement on these courses requires time and funding must be identified. Several research institutions have developed funded research fellowships to be taken as out of programme for research, which extends the time required to complete a training programme. Such fellowships offer an excellent opportunity to develop a deeper understanding of the subject. For some trainees, the fellowships act as a bridge directly into a PhD and a pathway towards academic National Institute for Health Research posts.
There is debate as to how molecular pathology is practised and at what level, with several models proposed for future training programmes.3 These include the delivery of morphomolecular training within the current five-year timeline by modifying the curriculum to include education on molecular medicine and by condensing current morphology teaching. Other options may be proposed, for example molecular pathology to become a specialty in its own right, with its own certificate of completion of training and a bespoke training programme. An alternative approach would be the development of a subspecialist area of interest within the existing training programmes. This latter approach is easier to manage within established career pathways.
Embedding genomics education to support clinical practice
To support the successful delivery of the Genome UK strategic vision, there is a requirement for workforce development and engagement with genomics through training, education and new standards of care.4 By recognising the need to upskill the healthcare workforce in genomics, considerations around framework, spending and policy have been prioritised.
Alongside primary care practitioners, midwifery and pharmacy, among others, the pathology workforce has been specifically acknowledged as a specialisation to be upskilled. The prospective workforce also needs to be trained in genomics.
The Genomics Education Programme (GEP) was a four-year endeavour within Health Education England. Funding of the GEP has subsequently been extended to continue their work in providing a coordinated national direction of education and training in genomics, and developing resources and educator toolkits to embed in pre- and postgraduate curricula.4,11 Alongside primary care practitioners, midwifery and pharmacy, among others, the pathology workforce has been specifically acknowledged as a specialisation to be upskilled. The prospective workforce also needs to be trained in genomics.
The infrastructure to support the embedding of genomics into clinical practice will be implemented by seven Genomic Medicine Service Alliances (GMSAs) that have been formed across England.12 Each GMSA will oversee the systematic embedding of genomic medicine within their region by engaging and establishing networks between NHS providers, including clinical and laboratory genetic services, primary care networks, Cancer Alliances, integrated care systems, Academic Health Science Networks, academia and other organisations. In addition, the Academy of Medical Royal Colleges has been commissioned by NHSE to ensure there is clinical engagement and oversight of the implementation of genomics into the NHS.13
The future of molecular pathology training
Current training does not adequately equip pathologists with the significant knowledge and skillsets required for the successful integration of genomic information with morphological pathology. However, it should be borne in mind that the majority of histopathology departments currently feel they lack the adequate staff numbers to meet clinical demands (Figure 1).
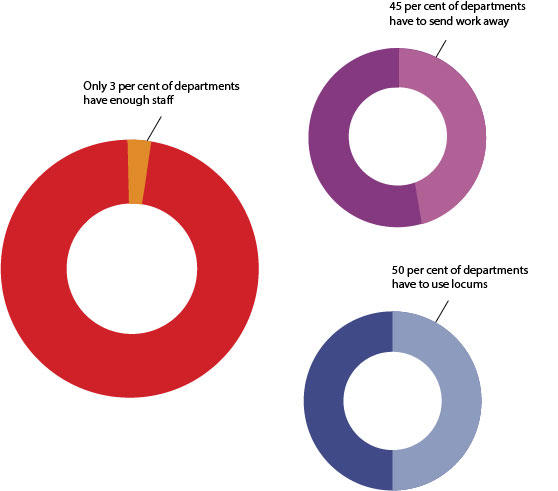
This is an issue that will need to be addressed, alongside upskilling the workforce to enable the specialty to reach novel clinical demands. Although the model by which training needs will be addressed remains unclear, what is certain is that equipping the pathology workforce to integrate modern genomic analysis with traditional morphology is essential for the enhancement of patient care, which will play a vital role in the UK’s position as a global leader in genomic healthcare.







