Excessive bleeding within the first 24 hours after childbirth, known as primary postpartum haemorrhage (PPH), remains the leading cause of maternal death in developing countries.1 In the UK, the risk of death from PPH is low (about one in 100,000 deliveries),2 however women who survive severe PPH suffer more long-term morbidities compared with other types of obstetric complications.3
There is no universally accepted definition for PPH. The World Health Organization defines PPH as a blood loss of ≥500 ml within 24 hours after birth, whereas the Royal College of Obstetricians and Gynaecologists classifies PPH into minor (500–1,000 ml) and major (>1,000 ml), with the latter being subdivided into moderate (1,000–2,000 ml) and severe (>2,000 ml).4 Over the last decade, the incidence of PPH has risen in developed countries (UK included) owing to increasing maternal age at time of delivery, multiple births, obesity and increased obstetric interventions such as caesarean section (Figure 1).5
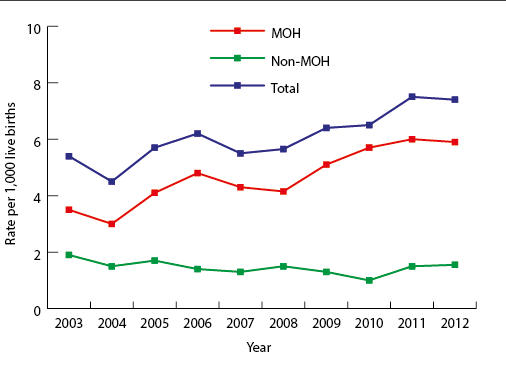
Over the last decade, the incidence of PPH has risen in developed countries (UK included) owing to increasing maternal age at time of delivery, multiple births, obesity and increased obstetric interventions.
The treatment for primary PPH requires a multidisciplinary approach. Interventions like uterotonic agents, surgical and radiological interventions, and haematological treatment are essential for controlling bleeding. In this article, we will briefly summarise some of the achievements from recent years on the haemostatic and transfusion management of PPH and highlight some ongoing challenges.
Transfusion management
Transfusion of blood components with red blood cells, plasma and platelets is an essential part of resuscitation of bleeding patients, with initial empirical use of these components being delivered as part of major haemorrhage protocols. These protocols allow for the delivery of blood components in fixed ratios, aiming to correct coagulopathy early and allow for the rapid issuing of blood through better communication between laboratory and clinical teams.6 However, we don’t know if ratio-driven transfusion in PPH improves outcomes, owing to the lack of randomised control trials (RCTs). Further, there is concern that this approach may expose women to unnecessary transfusion, particularly plasma.
Conventional laboratory-based clotting tests have slow turnaround times and point-of-care testing such as viscoelastic haemostatic assays (VHA) are now being used to guide targeted transfusion in PPH. The two most used devices are thromboelastography and rotational thromboelastometry, both utilising whole blood samples to assess coagulation and fibrinolysis. Currently, there is limited evidence supporting their use in guiding transfusion in PPH, as highlighted by two systematic reviews.7,8 In addition to the need for evaluating its cost-effectiveness, it is also important that VHA protocols are standardised prior to widespread application in routine practice.
The two most used devices are thromboelastography and rotational thromboelastometry, both utilising whole blood samples to assess coagulation and fibrinolysis.
Haemostatic drugs
Tranexamic acid
An antifibrinolytic agent that competitively inhibits plasminogen activation, tranexamic acid (TXA) is used widely to reduce blood loss by preventing clot breakdown (fibrinolysis). The benefits of TXA in PPH was demonstrated in the WOMAN trial, which was an international, double-blind, placebo-controlled study that randomised 20,060 women with PPH to intravenous TXA versus placebo in addition to usual care.9 Its results showed that TXA reduced the risk of death from exsanguination (RR: 0.81; 95% CI: 0.65−1.0; p = 0.045), with the treatment benefit being the strongest when TXA was administered within three hours of birth. Based on these results, TXA should be adapted into routine clinical practice and be given to all women with PPH.
Fibrinogen concentrate
Fibrinogen levels fall early in PPH and a low level of fibrinogen (<2 g/l) is an independent predictor of morbidity for women.10 The timing of when to replace fibrinogen in PPH remains unknown. A systematic review that evaluated the early use of fibrinogen replacement therapy in PPH identified two small RCTs (total of 299 women) that compared fibrinogen concentrate (FgC) with placebo on overall transfusion and both trials showed no benefits with FgC.11 Another similar RCT recently showed that FgC (versus placebo) did not reduce blood loss or transfusion needs in PPH.12 In all these trials FgC was administered when fibrinogen levels were >2 g/l, which might explain why there was no benefit with FgC.
There have been no studies on the use of cryoprecipitate in PPH, despite the component being the main source of fibrinogen replacement in many countries.
There have been no studies on the use of cryoprecipitate in PPH, despite the component being the main source of fibrinogen replacement in many countries. The systematic review identified only one ongoing pilot cluster RCT assessing the feasibility of administering cryoprecipitate early versus providing standard care to women with more severe PPH who require blood transfusion.13 There is a need to evaluate the role of cryoprecipitate in PPH, as well as compare cryoprecipitate versus FgC in PPH, particularly as the former contains additional factors like factor XIII, fibronectin, von Willebrand factor antigen and factor VIII, in addition to fibrinogen.
Recombinant activated factor VII
Licensed worldwide, recombinant activated factor VII (rFVIIa) is used in haemophilia A and B patients who have inhibitory antibodies against factor VIII or factor IX. Its use for management of PPH has mainly been through case report studies. In 2015, the first multicentre RCT compared the use of FVIIa versus standard care in 84 women with severe PPH who were unresponsive to uterotonics treatment. Results showed that rFVIIa reduced the need for specific second-line interventions such as interventional haemostatic procedures for blood loss and transfusions.14 However, this was a small study and not a placebo-controlled, double-blind trial. Large trials are needed to establish the safety and efficacy of rFVIIa for management of PPH.
Alternatives to blood transfusion
Cell salvage
To date, the largest multicentre RCT that has compared the efficacy and safety of cell salvage with no cell salvage is the SALVO study, which used cell salvage during caesarean section for women at risk of haemorrhage. The use of cell salvage in this trial showed a modest effect on overall transfusion rates (primary outcome) versus no cell salvage (2.5% vs 3.5%; 95% CI: 0.42−1.01; p = 0.056), and higher fetal maternal haemorrhage in RhD-negative women who delivered RhD-positive babies (25.6% vs 10.5%; p = 0.013).15 The additional cost of routine cell salvage use during caesarean was estimated, on average, to be £8,110 per donor blood transfusion avoided. Based on the findings of this trial, the authors concluded that cell salvage is unlikely to be considered cost-effective.15
Future research
Given the difficulties of undertaking large trials in a challenging environment, it is encouraging to see the increasing interest in haematological management of PPH with key achievements around better understanding of the role of TXA and the cost-effectiveness of cell salvage in this setting. Future research in haematological management of PPH should focus on determining: the optimum ratio of blood components; if a VHA-guide transfusion approach is better than a ratio-driven transfusion; the efficacy and safety of FgC versus cryoprecipitate; and the role of rFVIIa.





