Introduction to ZSL
The Zoological Society of London (ZSL) is a science-driven conservation charity that works to protect and restore wildlife in the UK and around the world. ZSL employs a wide range of pathologists, vets, microbiologists and scientists to perform post-mortem examinations of both captive and free-ranging wildlife to monitor the health of individuals and populations and investigate and respond to disease threats.
ZSL was founded in 1826 by luminaries including Sir Stamford Raffles and Sir Humphry Davy and quickly received a royal charter from King George IV. The Society opened the world’s first zoological gardens 2 years later, dedicated not to entertainment but to science – London Zoo. The zoo remained a private scientific collection, accessible only to members, until 1847 when it was opened to the wider public.
In 1931, a second site, Whipsnade Zoo, was opened to provide more space. In 1960, our research institute, the Institute of Zoology, was founded. Over the last 200 years, ZSL has worked to restore wildlife through vital conservation breeding programmes, establishing the first ever Red List of endangered species, opening the world’s first aquarium and reptile house, and discovering species previously unknown to Western science, such as the elusive okapi.

Pathology and ZSL
The wildlife health services team provides clinical and pathological services to ZSL’s zoo animals and conservation programmes. This includes a diagnostic service that performs approximately 800 post mortems every year, including about 100 mammals, 150 birds, 150 fish, numerous invertebrates and a small scattering of amphibians and reptiles. The largest animals, such as rhinoceroses and elephants, require many hours, heavy lifting equipment and large teams of pathologists, vets, nurses and technicians. Post mortems on the smallest animals, such as corals or small fish, may be done entirely via histopathology, as a whole carcass can fit on a single slide.
One of the major challenges for the wildlife pathologist is the lack of molecular tests to confirm suspicions of disease; even simple technologies such as immunohistochemistry are extremely difficult in many species owing to the lack of available or reliable antibodies. PCR tests are often not commercially available and must be specially designed for each pathogen of concern.
The plight of the Partula snail
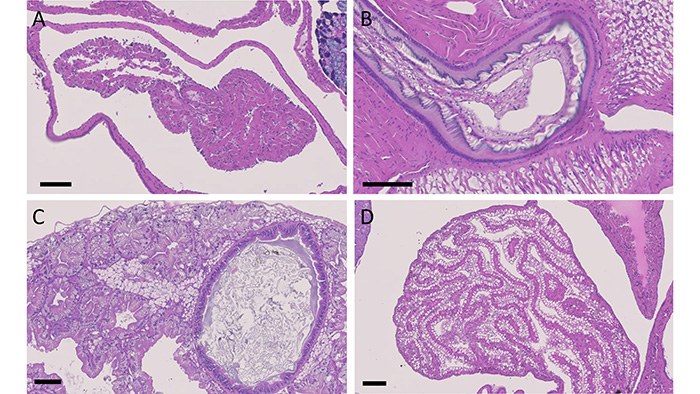
Partula is a genus of tree snails native to Polynesia, with multiple different species spread over the different islands (Figure 1). The snails have long been of interest to evolutionary biologists; their marked variation and speciation across the islands of the South Pacific is a textbook example of adaptive radiation.
Additionally, their shells were used by the Polynesians to make jewellery, with each island culture developing a distinctive fashion based on their local snail species. It was these ancient Polynesians that first began to translocate snails between islands, particularly Partula hyalina, which was prized for its bright white shell.
However, in 1967, giant African land snails were introduced to Tahiti as a potential food source. These snails quickly became pests and caused damage to crops, leading to a crude attempt at biological control when, in the 1970s, predatory rosy-wolf snails were released to predate the invasive land snails. The predators, however, turned out to much prefer eating the native Partula snails than the larger land snails; several species were quickly eaten to extinction.
In 1991, ZSL invertebrate keepers travelled to Tahiti and rescued the surviving members of several Partula species and, together with an international consortium, have been breeding them in captivity ever since.
Monitoring Partula snail health
Although captivity protects the snails from predators, it presents its own risks. The snails are kept in a biosecure facility, but there is always the risk that they may acquire novel pathogens either from other species of Partula or from other snails and invertebrates in the zoo, both captive and wild. An organism that co-evolved with 1 species of snail may be highly pathogenic in another; the close confines of their enclosures may allow pathogens to spread rapidly throughout a population.
This was the fate of Partula turgida, a species extinct in the wild that then became fully extinct when its captive population of 296 individuals slowly collapsed over 2 years. Post-mortem examination of 5 of these snails at ZSL revealed large numbers of microspodian parasites in cells of the gut, uterus and digestive gland (a liver-like organ).
Microsporidia is a diverse group of single-celled fungal pathogens (previously considered protozoa), characterised by a coiled germ-tube used to infect the host cell. The genera likely best known to medical pathologists are Encephalitozoon and Entercytozoon, which can cause disease in immunosuppressed patients; Encephalitozoon cuniculi is a very common parasite of rabbits and can be spread to humans via their urine. In P. turgida, the microsporidian was identified as a species of Steinhausia; this was believed to be the first definitively identified example of a species becoming extinct due to an infectious disease.

If the snails were to be released back into the wild with such a pathogen, it could have devastating consequences for these tiny, critically endangered populations. With most species weighing less than a gram, most of which is shell, clinical monitoring of the snails is almost impossible and is limited to observing changes in behaviour, food consumption and mortality. Thus, it falls to the pathology team to monitor for disease and screen the populations prior to release.
Post-mortem examination of dead snails is often an unrewarding experience. Algor mortis, the cooling of bodies after death, and refrigeration are useful retardants of putrefaction and autolysis in humans because all of our digestive enzymes and commensal bacteria are adapted to function at body temperature and thus are inhibited by cold.

Partula pathology
Dead snails are carefully dissected out of their shells with scissors and a small incision is made into the coelom (body cavity), which is swabbed for microbiology. The most apical loop of the snail, right up in the point of the shell, is carefully removed (snails can be gently ‘unscrewed’ out of their shell), providing a small sample of intestine from which impression smears can be made to look for parasites, whereupon the apex is stored frozen in case molecular identification is required. A small sample of the muscular foot is taken for DNA biobanking and the rest of the snail is fixed whole in formalin for histopathological examination.
Gastropod histology is markedly different from that of a human or any vertebrate, but the necessities of life mean that analogous organs are present, such as eyes, lungs, an intestinal tract, gonads, kidney and even a tiny 2-chambered heart. In some cases, fundamental physical laws mean that these organs resemble their mammalian counterparts – a heart will always be a chamber surrounded by muscle, a gut will always be a muscular tube with a thin epithelium.
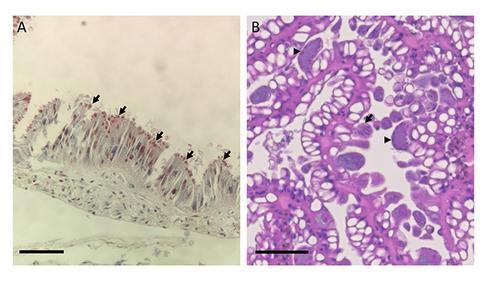
Other organs are less constrained and may appear completely alien, such as the tongue-like radula, a chitinous rasp used to scrape foods into the mouth (Figure 2). There is also significant variation on a cellular level, with some cells containing wide varieties of intracytoplasmic inclusions that can be impossible to distinguish from viral or parasitic inclusions without electron microscopy.
As well as microsporidia, a collaboration between pathologists and parasitologists at ZSL, the International Zoo Vet Group and the Royal Veterinary College has identified a novel species of Cryptosporidium that lives in the digestive gland, apparently without causing significant disease, and, recently, a coccidian organism that parasitises the epithelium of the kidney (Figure 3). Preliminary molecular studies have indicated that the coccidian is of the genus Klossia, which has previously been observed in other species of snail including those native to the UK, suggesting that this parasite may have been acquired from local species.

Conclusion
Since 1991, 15,000 snails have been reintroduced to Polynesia, with no evidence of disease introduction. Thanks to the work of ZSL and partner institutions, 11 species of Partula that were previously extinct in the wild have now returned to their native habitats, representing a major victory for conservation. If you would like to see the Partula snails and help fund their recovery by doing so, you can see them on display in the Tiny Giants building at ZSL London Zoo.