Maternal blood should be screened with now readily available technology to determine the RhD status of the fetus to support targeted anti-D prophylaxis of RhD-negative mothers carriying an RhD-positive fetus. In this article, Chris Elliot, from NHS Blood and Transplant, reviews the current status of implementation of this technique.
Anti-D immunoglobulin given to RhD-negative mothers after delivery of a RhD-positive baby suppresses the mothers’ immune response and the production of anti-D, thereby reducing the risk of haemolytic disease of the fetus and newborn (HDFN) in subsequent pregnancies.
The programme was further extended, with RhD-negative pregnant women being offered anti-D as part of routine antenatal anti-D prophylaxis (RAADP) to further reduce the risk of sensitisation. This is overall a modern success story with dramatic reductions in the incidence of HDFN due to anti-D.
While prophylactic anti-D is a very safe medication with adverse events occurring only very rarely, it remains a human-sourced product (which is, on occasion, in short supply). A monoclonal-derived, clinically effective antibody remains elusive. Accordingly, a more targeted approach to help administer prophylactic anti-D only when required will promote appropriate use.
Predicting the RhD phenotype
Over 30% of pregnant RhD-negative women carry a RhD-negative fetus, due to the incidence of the RhD-negative phenotype in the UK population. However, until relatively recently, it was not easily possible to determine the RhD status of the fetus until delivery. Routine prophylaxis was therefore given to all RhD-negative pregnant women with exposure of over 30% to an unnecessary intervention with a human-sourced product.
The advancement of molecular diagnostic technology has allowed the development of a test that detects the presence of fetal DNA gene in maternal blood and determines the presence or absence of an RhD gene in the fetus. The tests, thereby, accurately predict the RhD phenotype of the fetus from a gestational age of 11 weeks. This offers the opportunity to provide more personalised care to pregnant RhD-negative women by targeting use of prophylaxis only where the fetus has been shown to be RhD-positive.
An article in the July 2021 edition of the Bulletin summarised the role of the Serious Hazards of Transfusion (SHOT) haemovigilance scheme in the reporting of anti-D errors and optimising safety.
Implementing RhD testing
In 2016, the National Institute for Health and Care Excellence (NICE) recommended1 that fetal RhD screening of maternal blood samples using high -throughput technology should be adopted as part of the routine antenatal screening programme. This was reiterated in their 2021 review decision2 and testing was also recommended by the British Society of Haematology (BSH) guidelines.3
NHS Blood and Transplant (NHSBT) was able to offer hospitals access to this test by developing a high-throughput methodology at the Filton Blood Centre in Bristol. The cost of the test to hospitals was balanced by the reduced need to purchase prophylactic anti-D. The movement of samples was expedited through the existent transport system between hospitals and blood centres.
To retain efficiencies, the test was routinely undertaken by maternity teams between 16 and 24 weeks at appointments that were already within the routine antenatal care pathway. The increased cost of prophylactic anti-D has outstripped the cost of the fetal RhD screening test, which made this screen even more cost-effective than expected by the original NICE analysis.
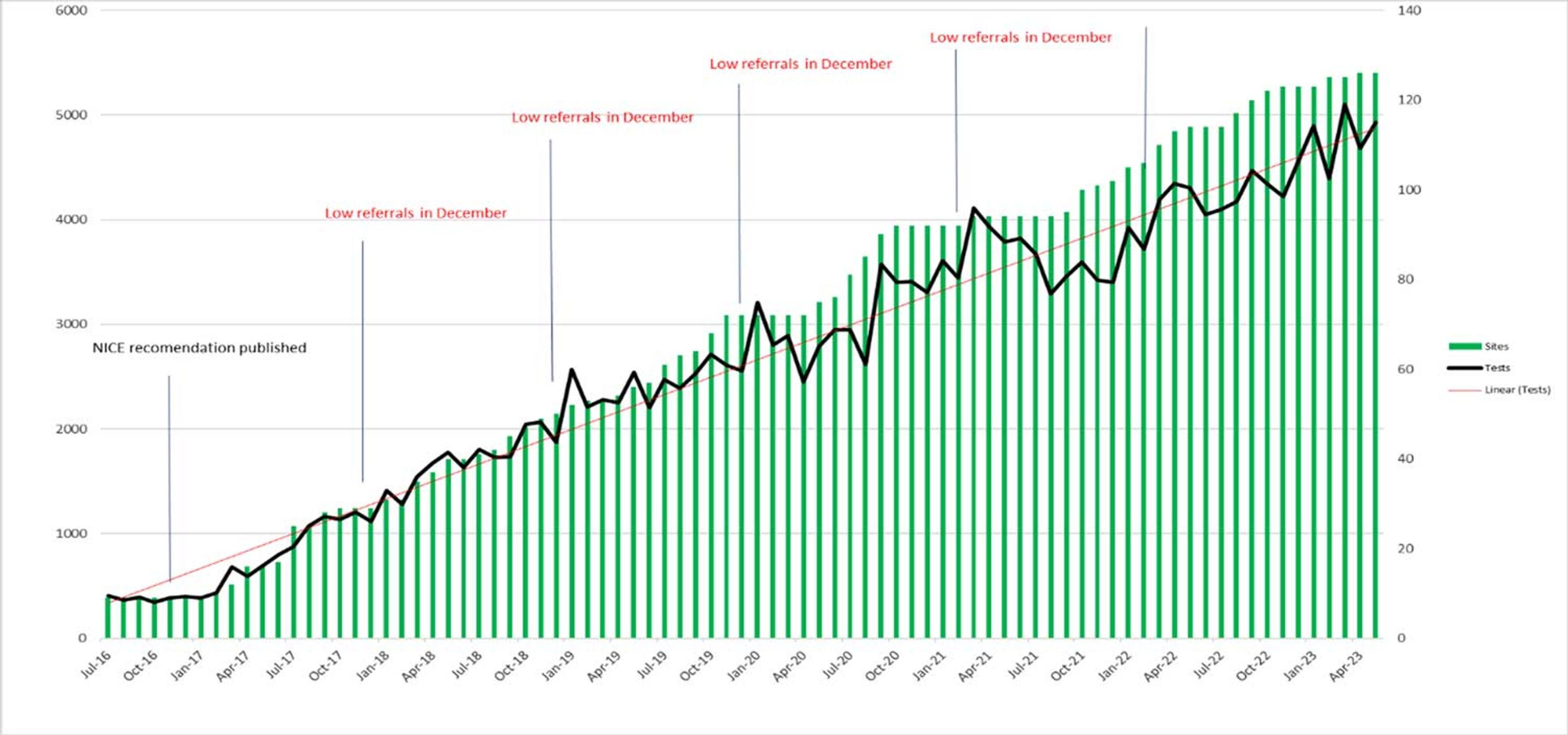
The uptake of fetal RhD screening by NHSBT has continued to grow over the last 7 years (Figure 1); over 60% of eligible pregnancies in England are now screened by this service. Of these tests, almost 60% return a positive result for RhD (Table 1). Welsh and Scottish hospitals only rarely send their samples to NHSBT for fetal RhD screening and, as yet, have no national provision of their own.
Table 1: Summary of fetal RhD screening tests performed by NHSBT from April 2017 to May 2023 (total of 184,532).
| Outcome of test | Number of tests with this outcome | Percentage of total |
|---|---|---|
| RhD-positive | 107,839 | 58.4% |
| RhD-negative | 67,596 | 36.6% |
| Inconclusive | 9,097 | 5% |
Advantages of fetal RhD screening
Aside from reducing unnecessary administration of anti-D as part of RAADP, the ability to discern whether the fetus is RhD-negative has additional benefits in the management of RhD-negative pregnancies.
- Aside from reducing unnecessary administration of anti-D as part of RAADP, the ability to discern whether the fetus is RhD-negative has additional benefits in the management of RhD-negative pregnancies.
- BSH guidelines recommend that, if anti-D is detected in the plasma as part of antenatal screening tests, quantitation should be performed to determine if the anti-D is likely to be prophylactic or immune.3 This becomes unnecessary once a fetus has been determined to be RhD-negative.
- If the fetal RhD status is known, then sending a cord or check group after delivery becomes unnecessary, reducing unnecessary tests in the newborn.
Barriers to adoption
So why hasn’t every maternity service or hospital blood bank introduced fetal RhD screening into routine testing?
Inevitably, the COVID-19 pandemic slowed the uptake of the test in some hospital antenatal services, but there is now a steady increase in adoption in units across England within their antenatal screening programmes.
Hospital transfusion laboratories and maternity units continue to experience significant staffing pressures, which limits their capacity to bring in new ways of working. NHSBT is working with these units to enable them to learn from the experiences of those already delivering fetal RhD screening to reduce the impact of adopting this new service into their antenatal pathway.
Endorsement by clinical guidelines
An article in the British Journal of Obstetrics and Gynaecology published in 2015 recommended that fetal RhD screening should be extended across all UK NHS services, supported by a commentary on behalf of the Royal College of Obstetrics (RCOG).4
There is a need for ongoing collaboration between relevant professional organisations to ensure updated and explicitly clear guidelines promoting recommendations for fetal RhD screening for all RhD-negative mothers. This includes guideline writing groups at the BSH and the RCOG. This needs to be supported by ongoing effective audit to demonstrate implementation together with the provision of appropriate information to RhD-negative mothers to promote informed consent and decision-making.
Future implementation
In the future, NHSBT will continue to support maternity and laboratory services across England in implementing fetal RhD screening into antenatal care pathways. An important development currently being piloted is electronic requesting and reporting of fetal RhD testing. This allows hospitals to send samples to NHSBT, with the request being sent electronically as opposed to on a paper request form. The result can then be sent back to the hospital electronically for direct integration into the hospital laboratory information management system (LIMS), without the need for transcription of the result into the LIMS.
This will help streamline the process, reduce manual transcription errors and hopefully reduce request rejection rates, which are currently running at about 5% and are usually due to patient demographic error, particularly incorrect estimated date of delivery. NHSBT is also willing to support the UK blood services in the devolved nations to implement fetal RhD screening.
Acknowledgements
Nicole Thornton from the International Rare Donor Panel, Erika Rutherford from NHSBT and all those providing the screening service at Filton NHSBT.