Cancer pathways
Pathology plays a pivotal role in cancer pathways, from diagnosis to treatment, as well as ongoing monitoring.
Nationally, there has been an increase in histopathology (part of cellular pathology) activity of 30% since 2018-2019; however, the consultant workforce has grown by only 8% over the same period.
Addressing workforce shortages across pathology, enhancing screening, and investing in a more integrated genomic testing service and in technologies such as AI and digital pathology are essential to improving cancer diagnosis and treatment.
The case studies below highlight the vital role of pathology in diagnosis and treatment of blood cancer, lung cancer and rare brain tumours in children.
Innovation in Diagnosis: One Test, Big Impact; George's story
Advances in haematology mean that patients with high platelet counts no longer always face invasive investigations to find out what’s wrong.
In the past, a bone marrow biopsy was often required to confirm a diagnosis. Now, in many cases, a single blood test can provide the answers.
A Cause for Concern
George Puddin, aged 76, undertook a routine blood test which revealed a high platelet count. His GP was unsure of the cause and referred him to local haematology clinic. Before his appointment, George searched online and spoke with friends, who all told him he would need a bone marrow biopsy; a procedure he was worried would be painful and difficult.
Faster Results, Less Worry
When George arrived at the haematology clinic expecting to need a biopsy, he was surprised to learn that all the necessary investigations could now be carried out with a simple blood test. A JAK2 test was performed, and within just a couple of weeks the results were available. George was promply diagnosed with essential thrombocythemia (ET), a type of blood cancer that affects the bone marrow, without the need for any invasive testing.
Because the results came through so quickly, George was able to start treatment straight away. Best of all, he avoided the invasive procedure he had been so worried about.
Better for Patients
Several months on, George’s platelet count is back to normal, and he is once again enjoying life as usual. For him, the real benefit was clear: one test, faster results, less worry.
Mary’s story; QuicDNA transforms lung cancer care with rapid diagnosis and treatment

The QuicDNA project is a transformative initiative taking place across six Health Boards in Wales. By incorporating liquid biopsies into lung cancer assessments, this technology offers a faster, less invasive method of gathering vital information.
Liquid biopsies analyse circulating tumour DNA (ctDNA) in a blood sample, supporting diagnosis and a quicker start to treatment. Ultimately, QuicDNA aims to improve outcomes for people with lung cancer.
Mary Simpson, a great-grandmother who loves walking and being outdoors, had been looking forward to resuming her active lifestyle after a second knee replacement in 2023. But by spring 2024, she began experiencing persistent leg pain, which was put down to a trapped nerve.
“I thought, brilliant, I can get back to doing some reasonable walking again. But that was when a pain in my leg began, and that was the beginning of all these problems,” Mary recalls.
A routine blood test revealed very high B12 levels, and she was sent for a CT scan. While on holiday in Cornwall, Mary received a phone call that set her on an unexpected path.
“A phone call at 9am on the Monday morning and I thought, golly, something is wrong for the doctor’s surgery to be calling at that time,” she says.
An appointment was made for her GP to call later, when she was told that the scan had revealed a small spot on her left lung. The day after returning home from Cornwall, Mary had an appointment for an MRI scan.
Just days before her 80th birthday, Mary was diagnosed with lung cancer and secondary bone cancer.
Although Mary praises her healthcare team, the news was a shock for her and her family. “Our daughter was with us when we were first told, and I think our whole family went into shock,” she says.
How QuicDNA transformed Mary’s care
Mary’s next steps included respiratory consultations and additional testing. As part of her assessments, Mary had a liquid biopsy blood test, through the QuicDNA lung cancer project.
This innovative test significantly shortened the time to treatment, providing critical information for her clinical team weeks ahead of traditional methods. Mary was able to begin targeted lung cancer treatment in September 2024, an entire month before tissue biopsy results, which would not have been available until October.
Dr Paul Shaw, Consultant in Clinical Oncology at the Velindre Cancer Service, highlights the profound clinical and emotional impact:
“Mary’s case demonstrates the transformative potential of rapid ctDNA analysis. The psychological impact of waiting for diagnostic results cannot be overestimated; this can be a source of very understandable concern for many patients and families who without ctDNA results may wait patiently for weeks at such a difficult time.”
This accelerated pathway allowed Mary to receive care from the oncology team much sooner, reinforcing the value of liquid biopsy as a game-changer in lung cancer diagnostics and treatment.
Positive outcomes and next steps
Mary felt an improvement almost immediately after starting treatment. The leg pain that disrupted her daily life, vanished. While she experienced some side effects, adjustments to her medication alleviated them.
In January 2025, Mary received encouraging scan results. “The tumour on my lung has shrunk a bit, and my understanding is that where the cancer has gone into my bone, it’s contained and hasn’t got any worse. I think I’m still in shock, it really knocks you sideways.”
Mary continues to have scans every three months and remains optimistic. She says, “You’ve got to stay positive. I’m quite a philosophical person and I take every day as it comes, which is all you can do.”
About QuicDNA and liquid biopsies
The QuicDNA project is paving the way for faster, more effective cancer care through innovative testing methods. Liquid biopsies offer a minimally invasive, rapid alternative to traditional diagnostics, enabling earlier intervention and better outcomes.
Learn more about the project here.
The vital role of pathology in saving babies with deadly brain tumours - Dr Matt Clarke
Devastating news
For a family in Italy, what should have been a happy and exciting occasion turned into the worst of nightmares. A baby was on the way but at a routine ultrasound scan, they received some devastating news; their unborn baby had an aggressive brain tumour. It is difficult to comprehend who awful this news must have been. A few days after their little girl was born, she underwent a biopsy of the brain tumour. The biopsy was examined by a neuropathologist who diagnosed it as a high-grade glioma, a very aggressive type of brain tumour with a very poor prognosis. The neurosurgeons then took her to theatre for a larger operation to remove as much of the tumour as possible. Operating on brain tumours requires a careful balance of trying to remove as much of the tumour as possible but at the same time, trying not to damage any of the important structures which may leave the patient with ongoing problems. Despite their best efforts, the neurosurgeons could not remove all of the tumour and so some of it was left behind. The remaining tumour cells therefore had the potential to continue growing and invading into this little girl’s developing brain.
How pathology research was able to help…
At this time, I was working on my PhD project which was focussed on understanding more about the molecular pathology of infant gliomas, a rare group of brain tumours affecting 2-3 babies in the UK each year and approximately 15 in Europe. I was undertaking this project at the Institute of Cancer Research, and was part of the Glioma Team, headed by Professor Chris Jones. We had been collaborating with national and international colleagues on this project, and therefore the team who were looking after this baby in Italy decided to send some of the tumour tissue to me for analysis to see if I could help understand more about it.
I was able to extract DNA from the tumour and then with the help of my colleagues in the Glioma Team, used a series of molecular techniques called DNA methylation profiling and fusion panel sequencing to understand more about the genetic alterations of this tumour. We were able to identify that it was a novel type of high-grade glioma (now called an infant-type hemispheric glioma) and also contained a solitary genetic alteration called an NTRK gene fusion. Importantly, drugs were available that could potentially target this change. I was able to report this back to the clinical team looking after this little girl; she was subsequently commenced on these drugs and they monitored her progress using MRI scans. Remarkably, the tumour began to shrink until eventually it disappeared altogether. She has been doing extremely well and is now at school.
A lasting impact
The results from my PhD were published in a scientific journal and I was able to travel to many different national and international meetings and conferences to show these results, including the story of this little girl. As a result, there is now a new chapter in the World Health Organisation (WHO) classification of central nervous system tumours focussed on this new tumour, and there are clinical trials in place for other children who are confirmed to have one of these tumours. There are now more and more success stories like this and it has shown how important pathology research can be to help understand more about brain tumours.
Tumours that show aggressive behaviours and have a poor outcome may have weaknesses that we can target, and pathology can help us do this.
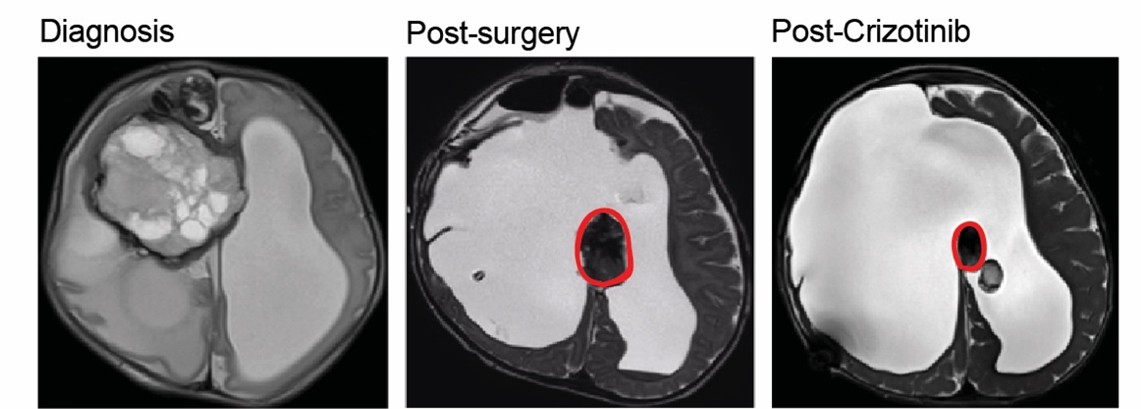
This picture shows a series of MRI scans of this baby. The first shows how extensive the brain tumour was at the time of diagnosis. The second shows a scan after the operation, and the remaining brain tumour is highlighted in red. The third shows the first scan after the baby was started on a drug treatment called Crizotinib; the tumour has started to shrink in size (1).
References
1. Clarke M, Mackay A, Ismer B, Pickles J, Tatevossian R, Newman S, et al. Infant high grade gliomas comprise multiple subgroups characterized by novel targetable gene fusions and favorable outcomes. Cancer Discovery. 2021;10(7):942-963.
